Imagine receiving a smidgeon of thymus gland along with a donated heart or kidney, to fool the immune system into accepting the transplant.
Authors of a new study argue that might be possible, based on their new proof-of-principle experiments.
The team from the Epithelial Stem Cell Biology and Regenerative Medicine lab at the Francis Crick Institute and University College London published their recipe for fashioning of a human thymus from stem cells seeded onto a bioengineered scaffold appears in Nature Communications. The rebuilt thymi began as stem cells from 33 patients aged 3 days to 11 years, collected during open heart surgery and then dissociated into suspensions of single cells.
Thymus and T cell education
In the thymus, T cells are “educated” early in life to distinguish self from non-self cell surfaces. They then leave the thymus to patrol for cells that have foreign antigens on their surfaces, such as virus-infected cells. These patrolling T cells also regard the cells of a transplanted organ as foreign and attack. In some cases this immune response can lead to rejection of the transplanted organ or even death of the recipient.
“It’s possible that we could overcome this by also transplanting a thymus regrown from cells taken from the thymus of the organ donor. We are confident that this may prevent the body attacking the transplant,” said senior author Paola Bonfanti.
Some skepticism on anti-rejection idea
Note that because Dr. Knoepfler viewed this statement as a somewhat speculative claim, he reached out to Dr. Bonfanti for an interview to clarify what she meant. The Q&A with her is included at the end of this article.
Knoepfler’s skepticism arose because the T cell portion of the immune system is already fully developed early in life. For this reason, the selection process against self-reacting T cells is complete way before any patients get an organ transplant. Therefore, even if a bioengineered thymus were transplanted along with the main organ that is the focus of the transplant (e.g. a heart or kidney), the patient recipient would still have their own T cells already out on patrol that could attack and reject the transplanted organ.
On an initial read of the paper it was difficult to imagine how the co-transplanted thymus could help prevent that.
Bonfanti provides some insights on what she is envisioning, including a tolerising role for special T cells called TReg cells from the cotransplanted thymus, in the interview, which you can jump to here.
Anatomy of a Thymus
The thymus is a soft, bilobed gland nestled behind the sternum and between the lungs. Connective tissue encapsulates it and strands divide it into lobules.
Disproportionately large in the fetus and during childhood, the thymus begins to shrink at puberty, as fat and more connective tissue replace the epithelium, interstitial cells, and lymphatic tissue that make up its bulk. The interior part is the medulla and the outside the cortex.
The epithelial cells secrete thymosins, which stimulate the specialization of thymocytes that originate much earlier as progenitor cells in the bone marrow. Only some thymocytes mature into T cells and exit the gland.
The thymus has long been a target for biomedical engineers, because of the importance of T cell education. If something goes awry, immunodeficiency or autoimmunity may result.
Thymic implants might help people with DiGeorge syndrome, who do not develop spleens, or people who have mutations in the gene that encodes transcription factor Foxn1, which impairs thymus development. Such mutations are best known in nude mice but have also been reported in an Italian village where several members of a seven-generation family have the condition, which causes congenital alopecia, underdeveloped nails, and severe infections that are lethal in early childhood. An autoimmune condition that might respond to a thymic implant is polyendocrinopathy-candidiasis-ectodermal dystrophy, which reflects mutations in the gene that encodes autoimmune regulator (AIRE), made in the medulla of the thymus.
For more on the thymus see a nice video from EuroStemCell below.
An Obscure Body Part
To biologists, the thymus is what puts the “T” in T cells. But historically, the gland has been something of an enigma.
The first description of the thymus is attributed to Rufus of Ephesus (98-117 AD), a Greek anatomist who, around the year 100, noted the resemblance to leaves of the thyme plant Thymus vulgarus. “Thymus” translates to “warty excrescence,” but “thymos” to soul or spirit, perhaps evoking proximity to the heart. In Italy the gland was called, in Latin, “aniella,” which means small soul, translated as “sweetbread.”
Galen of Pergamum (130-200 AD) noted shrinkage of the thymus with age, calling it an “organ of mystery,” and positing that it purifies the nervous system.
The thymus then seems to have vanished from recorded medical history for many centuries. It resurfaced when Berengario du Carpi (1466-1530), a lecturer of anatomy and surgery at the University of Bologna, meticulously sketched the gland. In the 1600s, Vesalius described the thymus as a cushion, and in the 1700s, the gland was thought to be a placeholder for the lungs in fetuses and newborns. Then in 1777, William Hewson, the “father of hematology,” from dissecting dogs and calves, deemed the thymus a lymph gland. Others considered the thymus vestigial, perhaps meaning that they had no idea what it actually did.
Then in 1961 French pathologist Jacques Miller described the role of the thymus in immunology of fostering self-tolerance. The epithelial cells make self-antigen precursors, which MHC molecules present. T cells that have receptors that do not react with the self antigens persist, and those that do recognize self die by apoptosis.
Of Stem Cells and Scaffolds
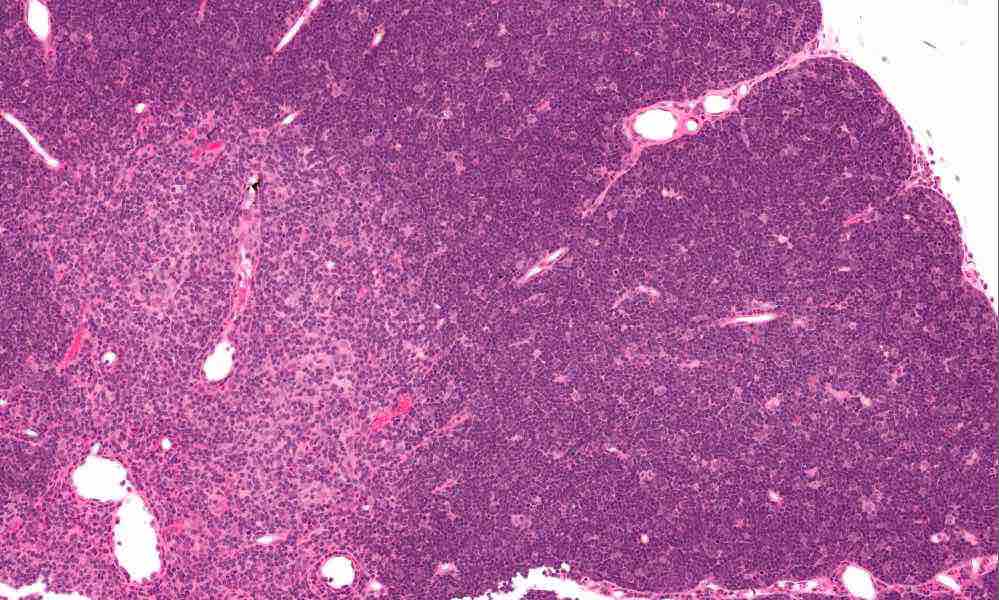
In the new study, when the researchers isolated and cultured thymus cells from the 33 patients, markers of being part of the medulla or cortex became less abundant as novel hybrid “clonogenic thymic epithelial-mesenchymal cells” emerged. The cells sport mesenchymal stromal cell markers. They persist over many divisions in culture and can be used to fashion a human thymus of sorts where T cells mature. You can see a histological, stained specimen of thymus in the image near the beginning of the post with cortex and medulla apparent.
The researchers nurtured many colonies of billions of cells; scale was important. “The fact that we can extensively expand thymic stem cells taken from human donors into large colonies is really exciting. It makes it possible to scale up the process with a view to build ‘human size’ thyme,” co-author Roberta Ragazzini said. The cultured thymi, when introduced into humanized immunodeficient mice, produced human T cells, in about 75 percent of attempts.
The three-dimensional structure of the thymus is also critical to educating T cells. Could the epithelial/mesenchymal hybrid cells repopulate a scaffold, mimicking a thymus?
Obtaining a scaffold wasn’t as straightforward as it is for other bilobed organs, like lungs, that share a blood supply. In the fetal thymus, connective tissue joins the lobes of the thymus, but the blood supplies are separate. So co-author Asllan Gjinovci invented a microvascular surgical technique that perfuses the entire gland through the carotid, tying off other vessels. This was done in rats, using iodine for visualization. X-ray microfocus-computed tomography revealed that the thymus extracellular matrix scaffold is rich in elastin but not in intermediate filaments, including keratins.
Six million injected human hybrid clonogenic TECs clung to the disembodied rat scaffold and proliferated, extending inward, festooned with markers of cell division but not of cell death – a good sign. When the researchers added thymic interstitial cells, outnumbering the TECs five to one, within 5 days the mass began to resemble a thymus of a 9-week fetus. The interstitial cells, then, are crucial for sculpting the gland.
Going in vivo: hope for future thymus transplants
In vivo experiments were telling too. The researchers implanted rat scaffolds under the skins of humanized mice, irradiated the animals, and then transplanted TEC, TIC, as well as hematopoietic stem cells from cord blood to possibly speed thymus development.
The scaffolds soon supported developing thymi, resembling the gland by 22 weeks. The cell structures even had telltale Hassall’s bodies, structures that are seen in human thymi but not in those of mice.
That wasn’t all – further experiments checked what was growing. The researchers dismantled the scaffolds, picked off grafts and dissociated them into single cells, and filed them through flow cytometry. Immunocytochemistry highlighted markers of T cell receptors, epithelial cells, mesenchymal cells, and angiogenesis. The T cells were marked with both human CD45+, common to all leukocytes, as well as human CD3+, the most abundant T cell co-receptor, which activates helper as well as cytotoxic T cells. Bone marrow from the same mice not given the scaffolds made mostly myeloid and B cells.
In a final experiment in this elegant series, humanized nude mice without thymi were given empty scaffolds, devoid of T cells, but that had human thymic mesenchymal stromal cells. Soon, human CD3+ T cells appeared in the peripheral blood, and tested positive for expression of 11 genes indicating T cell receptor activation.
Q&A between Knoepfler and Bonfanti
PK: I am unclear on the possible mechanism by which co-transplantation of a thymus with another organ (e.g. a heart or kidney, etc.) would potentially prevent rejection, as you indicated in your quote in the media. Could you clarify why you think this would be possible?
PB: In the quoted text, I also clarified that this will be a future application and not an immediate development. The rebuilt human thymus now allows us to dissect and study the tolerogenic mechanisms in humans for which a model system was missing.
Both organ rejection mechanisms and the cellular and molecular key players that orchestrate tolerance induction have been only partially elucidated and are still largely unknown in humans. With our bioengineered thymus, we have now the possibility not only to understand better these mechanisms but also to re-assemble ad hoc both the cellular and molecular drivers to instruct conventional and unconventional T cells for specific immunological purposes such as in the future a tolerising organ to donor’s tissue and organ transplantation.
PK: In my understanding, the typical adult organ transplant recipient would have plenty of their own mature T cells patrolling for foreign antigens and that could attack a transplanted organ too, regardless of the presence of a 2nd thymus. How would having a 2nd thymus that is a match to the transplanted organ prevent the existing T cells of the recipient from attacking the organ?
PB: In the first instance, the adult organ transplant recipient would receive the standard immune suppression therapy that will be needed until the co-transplanted thymus will mature and become able to develop its tolerogenic activity (e.g. production of Treg). Therefore, a progressive reduction and final elimination of life-long immune suppression may be possible. In our work, we have already demonstrated that a murine and human thymus can co-exist in a recipient and develop independently human T cells from HSC.
PK: It seems more likely to me (a non-immunologist) that your work has more clinical implications for patients that lack a thymus or have thymic dysfunction than by impacting patients getting organ transplants. Am I missing something?
PB: I fully agree that in the short-term, athymic patients would be the first to benefit of the translation of the reconstituted thymus. They can accept more easily transplants for their lack of T cells and our approach has the potential to be safer than their current therapy.
Dear Paul,
I am intrigue by the fact that new thymus will regard transplanted organ form donor as self, but how would they react with recipient (host) cells? they might regarded host cells as foreign? and attack host organs?
As it was explained to me by my hematologist a few years ago, as an older marrow recipient with chronic GVHD, I don’t have enough thymus cells to completely tolerance the T-cells produced by my donor’s marrow stem cells. And this will only get worse as more and more of the thymus tolerancing cells die off, which is inevitable with age. Just wondering if fortifying one’s own waning stock of thymus cells with implantation of induced ones might be helpful in tolerancing more of the antagonizing donor T-cells?
Nice article, this one. Thank you for publishing it!
The scarcity of complete DiGeorge syndrome probably led the researchers for a more common application of their principle. This being said, there is probably a need for DGS, complete or not. If the thymus fiction is just decreased, then a method similar to that proposed for transplant, i.e, developing tolerance may be applied.