Scientists studying genetics are both excited and worried about a powerful, new technology called “gene drive“. Some have been raising serious concerns about gene drive and in certain cases calling for proactive regulation, which is unusual in science.
This method is so powerful because it is designed to induce genetic changes in an entire population in a relatively extremely short period of time compared to natural evolution and is self-propagating. The most talked about form today is a type powered by CRISPR-Cas9 gene editing technology.
In principle, this kind of genetic modification engine can be used both in research in the lab and out in the world depending on the desired application. During my 25 years in science, I’ve rarely seen scientists so excited and also this unsettled about a single technology. While potential heritable genetic modification of human individuals via CRISPR is rightly generating substantial discussion, gene drive warrants increased attention and discussion because of its broad power and self-propagating nature.
Within the lab, this technology may make for more rapid, affordable, and in some cases elegant genetic experiments in model organisms. For instance, in mice the same kind of genetic experiments that could take years otherwise, may only take a few months if gene drive is utilized. This strongly resonates for me as a mouse genetics researcher. It can also be used in other model organisms such as fruit flies.
For hypothetical applications outside the lab, gene drive would take us into uncharted territory. It has been proposed to have great potential for health such as by targeting the mosquitoes that transmit malaria to humans and in agriculture by wiping out pests. A number of other hypothetical gene drive-based applications intended for real world implementation have been discussed. The  only requirement is that an organism reproduce sexually.
only requirement is that an organism reproduce sexually.
It works in a general sense by making a mutant gene be inherited more often than normal through an entire population. An engineered gene in a nuclease-driven gene drive system of the type powered by CRISPR-Cas9 might be inherited almost 100% of the time.
The more specific mechanism by which such a nuclease-driven drive works is by creating a mutant form of a gene that then itself also has the power (via CRISPR-Cas9 for instance) to change any additional WT copies of the gene that are present into drive mutants as well. In other words, a gene drive mutation has the ability to spread itself at the expense of normal WT copies of the same gene. For instance see Figure 1 from an intriguing gene drive paper from George Church’s group showing the possible spread of such a drive as represented by green mosquito symbols.
Eventually as gene drive continues there aren’t many if any WT organisms left anymore. Much of the subsequent mating may occur between mutants and all offspring are mutants. The selfish gene drive wins out and in evolutionary terms this happens at warp speed. In a sense it is a genetic chain reaction and one that it might be difficult for us scientists to control out in the field.
In the lab such a change may save researchers months or years of work compared to using now considered to be old-fashioned methods. In the real world, a gene drive could complete a genetic change in a population over a period of only months or years that might have naturally taken evolution millions of years. Of course, due to the artificial nature of this genetic change, something similar may never have occurred due to natural evolution. Imagine for real world applications if you could change a wild population of mosquitoes genetically such that they cannot be infected by Malaria and in turn they cannot infect people? Millions of lives could be saved.
Sounds amazing, right?
However, scientists are at least as worried about gene drive as they are excited.
Attempts at using gene drive to make ecosystem-wide genetic changes could prove extraordinarily risky for a variety of reasons. The first level of concern is over the sheer power and potential for speedy population-wide changes via fairly basic gene drives that hundreds or thousands of researchers could easily make in their labs today. The fact that gene drives are remarkably simple to engineer contrasts with the enormous complexity of how biological and genetic systems function as well as the possibility of unexpected and enormous negative consequences to gene drives run amok. For instance, if a problem manifested in rapidly reproducing, mobile insects due to use of gene drive, there might well be almost nothing that scientists could do to stop the gene drive chain reaction from spreading.
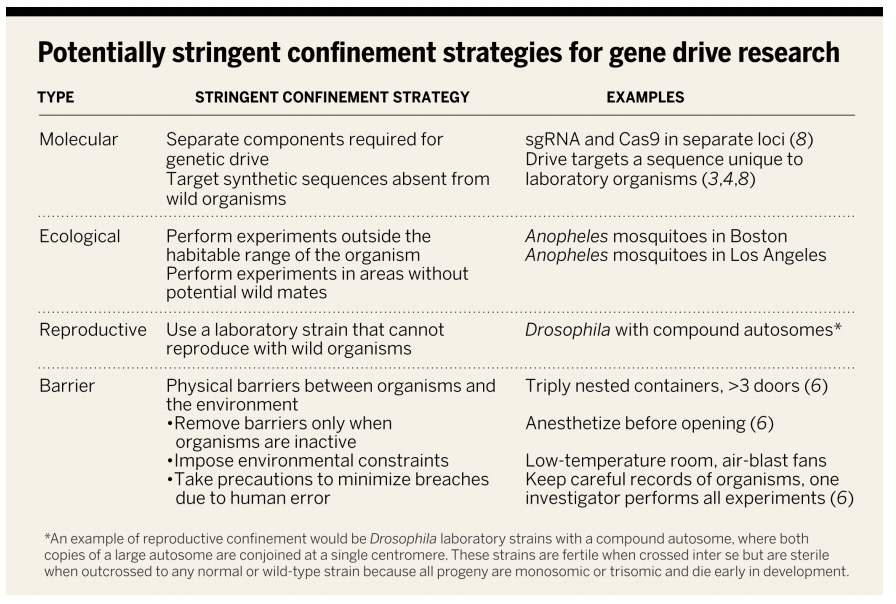
Even gene drive work intended to be limited to the lab has people very concerned. Model organisms such as fruit flies or mosquitoes that bear such a drive and are part of lab experiments could escape into the real world and mate with their wildtype counterparts. One analogy for this is a potentially toxic, genetic “spill” that is in a sense infectious and self-perpetuating. Many bio-containment and other precautions have been proposed to avoid this situation by keeping such organisms in the labs where they belong, but accidental release of gene driven organisms is a very real possibility.
Enabled by the simplicity and power of CRISPR-Cas9, many labs around the world may start making gene drive organisms without being fully aware of the risks. Akbari and colleagues have made a table (above) summarizing some proposed measures put forth collectively by the field to try to reduce this kind of risk as well as risks of actual intentional experiments in the field.
I recently talked with leading geneticist Harmit Malik, who I first met when I was a postdoc at the Hutch in Seattle, about concerns over gene drive. I hope to post this discussion in the near future. His perspectives on this important topic are very insightful and interesting.