There’s a new paper out this week in Cell from the Zaret lab that is very exciting.
In this manuscript, Facilitators and Impediments of the Pluripotency Reprogramming Factors’ Initial Engagement with the Genome, Zaret’s team explores how Myc and Oct4, Sox2, and Klf4 (OSK) behave at the genomic level during cellular reprogramming.
What are the most exciting and interesting points of this paper?
The team finds that Myc and OSK behave in some unexpected ways. For example, Myc greatly enhances the binding of OSK to the genome. In addition, Zaret’s group finds that OSK also act as pioneer factors for Myc, bringing it into chromatin. So OSK bind and recruit Myc, which then enhances their binding to genomic targets. Sort of a molecular mutualism, if you’ll buy that analogy. See their model below.
Interestingly, large domains of H3K9me3 impede reprogramming. Somehow OSK and Myc successfully overcome and/or limit these domains of H3K9me3, to trigger reprogramming. This fits with a longstanding model (e.g. see these papers: here (key paper), here and here) in the Knoepfler Lab of Myc being a global facilitator of euchromatin, a type of chromatin that is generally depleted for H3K9me3. Also fitting with the notion of heterochromatin inhibiting iPS cell formation is the fact that Zaret’s team also found that knocking down the histone methyltransferases responsible for H3K9 trimethylation seemed to improve reprogramming efficiency.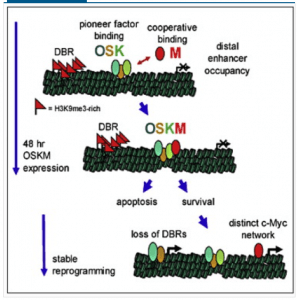
It is also intriguing that they found that during reprogramming the most important Myc binding site is not the canonical Myc E-box CACGTG, but a slightly different E-box sequence: CACATG. This really should be getting Myc folks to think more broadly about how Myc may bind DNA differentially in a context dependent manner.
In this paper they also found an intimate connection between OSK and Myc and apoptosis. Monkeying around with core epigenetic machinery may well trigger death in most cells so iPS cell formation likely selects for cells that can overcome that trigger. Such cells are as a result perhaps more prone to behave in an undesirable fashion that could in some circumstances ultimately lead to neoplastic activity.
Key open questions for future study.
Is related machinery is at work during oncogenic transformation stimulated by Myc or other oncogenes? Do OSK contribute to cancer as seems likely? My lab’s recent paper finds that oncogenic transformation and iPS cell formation are related (although distinct) processes. It is also notable that in neuroblastoma that Myc induces expression of other pluripotency factors including Klf4.
Do OSK play a role in transcriptional repression by Myc during reprogramming? Many studies including the first double Myc knockout in ES cells by lab (see here) indicate that a key function for Myc in maintaining pluripotency is repression of differentiation-associated gene expression. Do OSK influence that Myc function?
How do Myc and OSK counter the mega domains of H3K9me3? What’s the mechanism?
Overall, I believe that this wonderful Zaret lab paper provides a much-needed window into how Myc and OSK function during early reprogramming. You can’t answer every question in a single paper, not even a Cell paper, but this team did a great job.