Ready for the latest recommended weekly reads in the world of stem cells and the regenerative medicine space including a bunch of important new FDA posts & changes?
This post has quite a lot on the FDA since it had a very big week with several new items of major importance to the cellular and regenerative medicine arena. I’ve linked to each announcement below with the agency’s title of the announcements. Underneath I provide some analysis and ask questions.
I’ve also included some other stem cell news and exciting papers too as usual, which I’ll start with here.
Hair stem cells and goosebumps linked
Each year I am part of a team that teaches histology (technically “Cell and Tissue Biology”) here at UC Davis School of Medicine. As part of this I give a number of lectures including one on Integument, which is all about skin and associated structures. Certain details always seem to strike students as particularly interesting, including the arrector pili muscle, which gives us goose bumps by raising hairs up.
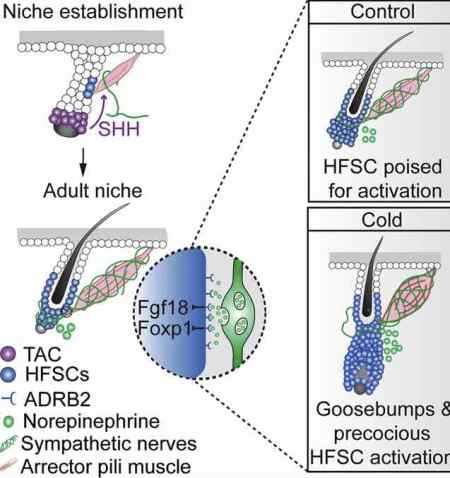
Now a new Cell paper reports an unexpected link between this tiny muscle that gives us goose bumps and hair stem cells. The paper is entitled, “Cell Types Promoting Goosebumps Form a Niche to Regulate Hair Follicle Stem Cells.“ See the article’s graphical abstract above.
In a nutshell, the team found that there’s a unique niche in which arrector pili muscle cells, nerve cells, and hair follicle stem cells interact, and cold/activation of the muscle can lead to more stem cells. This was observed in mice so it’ll be important to see if the same mechanism occurs in people. Here’s how they wrap up their abstract:
“Our results reveal a reciprocal interdependence between a regenerative tissue and its niche at different stages and demonstrate sympathetic nerves can modulate stem cells through synapse-like connections and neurotransmitters to couple tissue production with demands.”
The New Atlas also covers this new paper with some helpful points and quotes from the authors including this:
“We could really see at an ultrastructure level how the nerve and the stem cell interact,” says Ya-Chieh Hsu, co-lead author of the study. “Neurons tend to regulate excitable cells, like other neurons or muscle with synapses. But we were surprised to find that they form similar synapse-like structures with an epithelial stem cell, which is not a very typical target for neurons.”
One could imagine this research may have implications for stem cell baldness treatments that involve modulating that niche.

What can MSCs really do clinically and how to get there?
From Jeff Karp and co-authors comes, Shattering barriers toward clinically meaningful MSC therapies.
It’s a great read and bluntly discusses obstacles, but I’m a little less convinced that bioengineering can shatter the key obstacles to translating MSCs more broadly. Here’s one of the reality check quotes:
“most of the clinical-stage MSC therapies have been unable to meet primary efficacy end points. The innate therapeutic functions of MSCs administered to humans are not as robust as demonstrated in preclinical studies, and in general, the translation of cell-based therapy is impaired by a myriad of steps that introduce heterogeneity.”
Their schematic (above) is just great!
Autophagy and pluripotency
From Science comes Chaperone-mediated autophagy regulates the pluripotency of embryonic stem cells. A key quote from the pub highlights the main finding, “core pluripotency factors OCT4 and SOX2 suppress chaperone-mediated autophagy (CMA), a selective form of autophagy, until the initiation of differentiation.”
FDA drops many new items in cell therapy space.
FDA Extends Enforcement Discretion Policy for Certain Regenerative Medicine Products
Frankly, I don’t understand why the agency just extended it’s original three-year “grace period” of a sort or as they call it “Enforcement Discretion Policy” period. Otherwise this period would have ended in just a few months in November.
In the statement mentioning the 6-month extension they cite the COVID-19 pandemic as a general reason for the extension, but don’t say more about the need for the extension. Is it to allow for good citizen biotech and other sponsors to come into compliance? Is there a risk of giving not-so-good citizen stem cell clinics and suppliers more leeway to market illegal offerings for profit? Since the FDA is so much more on the ball with the clinics and suppliers, I hope this extension doesn’t slow down enforcement in the predatory clinic arena. The agency specifically does say in the announcement that, “this policy was never intended to provide a cover for bad actors.”
Regulatory Considerations for Human Cells, Tissues, and Cellular and Tissue-Based Products: Minimal Manipulation and Homologous Use
This announcement is about updated minimal manipulation and homologous use guidelines. The actual guidelines are here, which are a tough one to get through with 28 pages. The announcement mentions that the updated guidelines reflect the new enforcement period end date (mentioned above), but I haven’t figured out yet if there is more to this update or not. Stay tuned. Let us know if you have found anything else new in there.
Consumer Alert on Regenerative Medicine Products Including Stem Cells and Exosomes
This contains substantial new information from the FDA.
The tone of the language suggests a heightened sense of the risks to consumers. The inclusion of exosomes also reinforces the sense out there that marketing of unproven, even illegal exosome products has become a major problem.
Potential Risks of Treatment with Unapproved Regenerative Medicine Products
This article comes from CBER Director Dr. Peter Marks. I find it notable that Dr. Marks refers to unapproved stem cell and related regenerative products as “illegal” in this passage:
“Several times in recent years, the FDA warned consumers who have been treated with, or who are considering use of, unapproved stem cell, exosome, or other products marketed as regenerative medicine products, about their potential risk. These illegal products are often marketed by clinics under the umbrella term of regenerative medicine as being safe and effective for treatment of a wide range of diseases or conditions (e.g., Alzheimer’s disease and other neurologic disorders, orthopedic conditions), even though they have not been adequately studied in clinical trials. And more recently, some of these clinics have been marketing or distributing their unproven products to treat complications related to COVID-19 – claims that are not based on adequate clinical data.”
You’d think that illegal products would spark quick, robust activity. While we have seen far more activity by the FDA in recent years in regard to regenerative clinics, much more needs to be done and soon.
He also references an FDA video (below) warning about unapproved stem cell therapies from last September that somehow I hadn’t run across before. It’s an interesting watch, especially as it is very blunt about the status of unproven stem cells.
Blast from the past: zombie stem cells
Wanted both dead & alive: amniotic stem cell clinics sell zombie cells?
My post from 4 years ago on the then small but growing problem of birth-related clinics and suppliers. This sector of the regenerative space has become even more of a train wreck with many patients harmed and many more having lost collectively millions of dollars to at best sketchy therapies.
Thanks for explaining the concept of goosebumps thoroughly and I am fully sure hair are responsible for giving us goosebumps.
I’ve seen Dr. Peter Marks present a few times on the illegal stem cell market. It’s very clear he’s frustrated with the situation, and wants more to be done, but that action within the FDA structure makes it… difficult. He’s clearly concerned about patients being subject to effective fraud and a lack of enforceable reporting on adverse events. He’s always a great speaker. Perhaps you can do a blog interview with him some day soon.